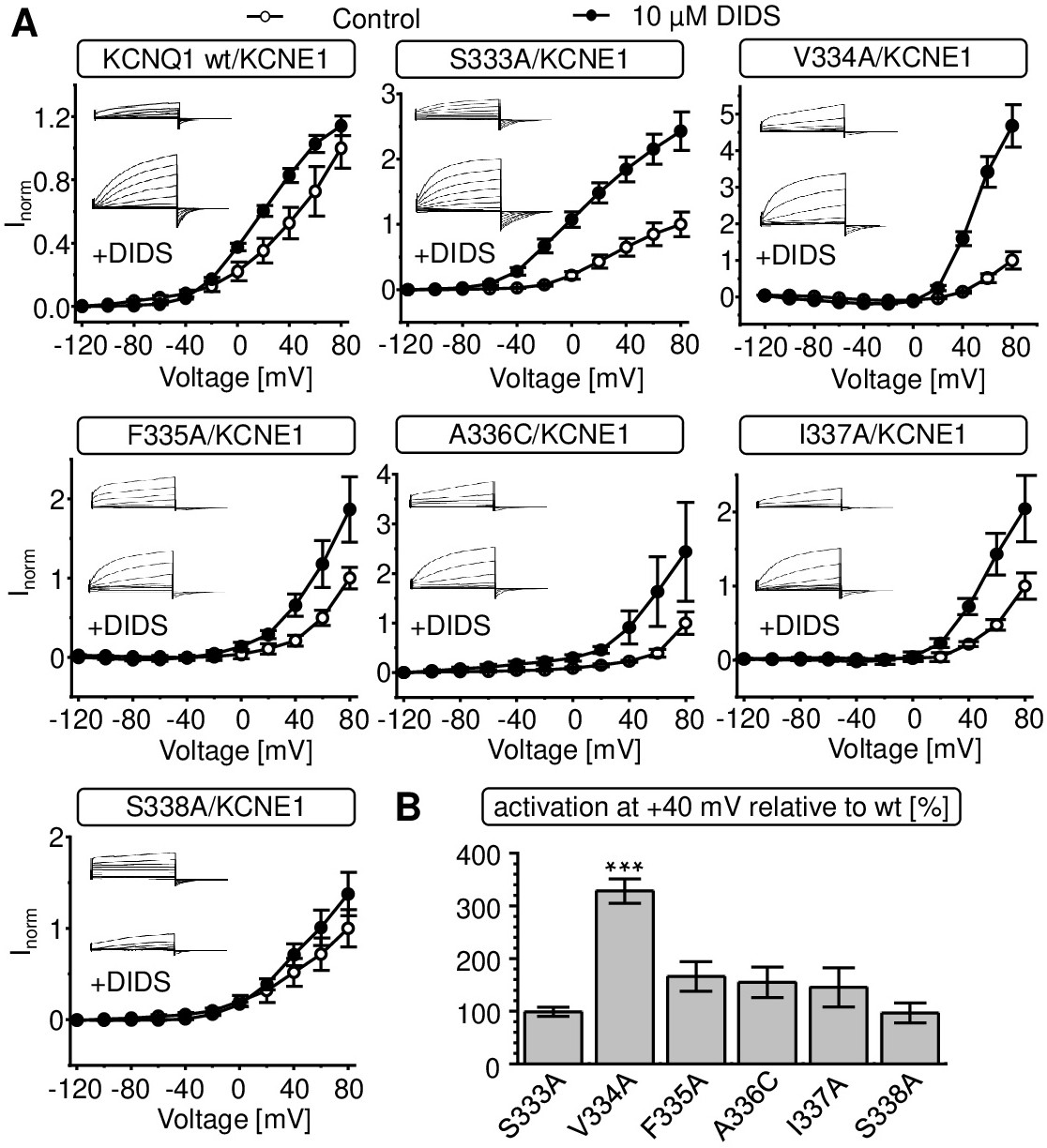
Fig. 4. Influence of KCNQ1 pore domain mutations on DIDS-effect on voltage-dependence of channel activation and change in current amplitude. A. Effect of 10 ÁM DIDS on the current-voltage relationship of wildtype and mutant KCNQ1/KCNE1 channels (KCNQ1-KCNE1 - ratio 1:2). Tail currents were recorded at -120 mV and additionally normalized to the averaged normalized tail current at +80 mV after application of ND96 containing 0.1 % DMSO instead of DIDS (voltage pulse-protocol (2) in methods section). Similar normalized tail currents after application of ND96, 0.1 % DMSO served as control (Q1 wt n = 4-6; S333A n = 4; V334A n = 3; F335A n = 9-13; A336C n = 7; I337A n = 11-14, S338A n = 3-4, ▒ SEM). Representative currents are shown as small inlay representations. B. Activation of wildtype and mutant KCNQ1/KCNE1 by 10 ÁM DIDS. Activation was defined as percent change in current amplitude at the end of the +40 mV depolarizing test pulse, using voltage pulse-protocol (1) described in methods section (n = 3-14, ▒ SEM). Activation (DIDS-stimulation) of mutant KCNQ1 coexpressed with KCNE1 at a ratio of 1 to 2 was normalized to activation of oocytes coexpressed with wildtype KCNQ1/KCNE1 from respective batches to minimize variation by preparation. Data were tested for significance using unpaired t-test and *** indicates p = 0.001.